Performing integrative analysis on scRNA-seq data and 10x Visium data of Human Lymph Node with STEP¶
This tutorial showcases the workflow of integrating two modalities with STEP where they are firstly co-embeded into a shared latent space; then the spatial domains(and subdomains) are identified based on the obtained embedding; subsequently, the cell-type composition of each spot is inferred based on the identified domains and according to the embedding in the shared latent space.
Loading packages¶
[1]:
import torch
import scanpy as sc
import numpy as np
import seaborn as sns
import pandas as pd
import matplotlib.pyplot as plt
import os
from step import crossModel
from step.utils.data import plot_posterior_mu_vs_data
sc_file = './results/lymph/human_lymph_integrated.h5ad'
sc_pth = './results/lymph/human_lymph_integrated.pth'
st_pth = './results/lymph/human_lymph_st_decoder.pth'
st_file = './results/lymph/human_lymph_filtered.h5ad'
if not os.path.exists('./results/lymph'):
os.makedirs('./results/lymph')
Load data¶
This data loading part refers to the tutorial of cell2location
[2]:
adata_ref = sc.read(
f'./data/lymph_sc.h5ad',
backup_url='https://cell2location.cog.sanger.ac.uk/paper/integrated_lymphoid_organ_scrna/RegressionNBV4Torch_57covariates_73260cells_10237genes/sc.h5ad'
)
[3]:
adata_vis = sc.datasets.visium_sge(sample_id="V1_Human_Lymph_Node")
adata_vis.var_names_make_unique()
[4]:
adata_vis.var['SYMBOL'] = adata_vis.var_names
adata_vis.var.set_index('gene_ids', drop=True, inplace=True)
[5]:
adata_ref.var['SYMBOL'] = adata_ref.var.index
# rename 'GeneID-2' as necessary for your data
adata_ref.var.set_index('GeneID-2', drop=True, inplace=True)
# delete unnecessary raw slot (to be removed in a future version of the tutorial)
del adata_ref.raw
Initialize the STEP instance¶
Initialization of STEP instance requires set the following parameters: - sc_adata: Anndata of scRNA-seq data - st_adata: Anndata of spatial data - n_top_genes or geneset: using hvgs or specified geneset - batch_key: a column name of sc_adata.obs specifying the batches in scRNA-seq data - class_key: a column name of sc_adata.obs specifying the cell-type annoations in scRNA-seq data - decoder_type: distribution(zinb or nb, default zinb) to model gene expression
data.
Other parameters are about model settings: - module_dim: dimension of final embedding - hidden_dim: dimension of hidden layers - n_moudles: number of gene modules - dec_norm: normalization layer used in decoder, - ‘layer’: LayerNorm - ‘batch’: BatchNorm - None: not using any normalization layer - n_dec_hid_layers: number of hidden layers used in decoder.
[6]:
proc = crossModel(
sc_adata=adata_ref,
st_adata=adata_vis,
n_top_genes=2000,
layer_key=None,
batch_key='Sample',
class_key='Subset',
module_dim=30,
hidden_dim=64,
n_modules=32,
decoder_type='nb',
dec_norm=None,
n_dec_hid_layers=1,
# model_checkpoint=sc_pth,
)
not log_transformed
Adding count data to layer 'counts'
Dataset Done
[7]:
proc.adata.obs['Sample'].value_counts()
[7]:
BCP005_Total 13323
BCP006_Total 4531
4861STDY7462253 4286
4861STDY7462255 4283
V1_Human_Lymph_Node 4035
4861STDY7528597 3888
BCP009_Total 3867
4861STDY7462256 3852
4861STDY7528600 3594
4861STDY7528599 3172
4861STDY7135914 3126
4861STDY7462254 3085
4861STDY7528598 2961
4861STDY7135913 2941
BCP008_Total 2914
Pan_T7935494 2876
BCP003_Total 2083
BCP002_Total 2002
Human_colon_16S8000484 1308
4861STDY7208413 1228
4861STDY7208412 1130
Human_colon_16S7255678 1125
BCP004_Total 985
Human_colon_16S7255677 700
Name: Sample, dtype: int64
Co-embedding two modalities¶
In this process, the goal is to extract the information from transcripts, also eliminate the technical/batch effects in the meantime, so the spatial data is not differentiated from scRNA-seq data by ignoring the spatial locations.
The following running parameters
tune_epochs=200,
need_anchors=False,
beta1=1e-2
are somewhat special: only when STEP is required to refine the model with cell-type annotations, where need_anchors=True is set, the tune_epochs is valid and control the number of iterations of the refine process. The beta1 is the coefficient of KL-divergence, the adjustment of this parameter can result in the change of the degree of embedding’s “dispersion”: higher values result in the lower degree of “dispersion”.
[8]:
proc.integrate(epochs=400,
batch_size=2048,
split_rate=0.2,
tune_epochs=200,
need_anchors=False,
beta1=1e-2)
Performing category random split
Training size for 4861STDY7135913: 2352
Training size for 4861STDY7135914: 2500
Training size for 4861STDY7208412: 904
Training size for 4861STDY7208413: 982
Training size for 4861STDY7462253: 3428
Training size for 4861STDY7462254: 2468
Training size for 4861STDY7462255: 3426
Training size for 4861STDY7462256: 3081
Training size for 4861STDY7528597: 3110
Training size for 4861STDY7528598: 2368
Training size for 4861STDY7528599: 2537
Training size for 4861STDY7528600: 2875
Training size for BCP002_Total: 1601
Training size for BCP003_Total: 1666
Training size for BCP004_Total: 788
Training size for BCP005_Total: 10658
Training size for BCP006_Total: 3624
Training size for BCP008_Total: 2331
Training size for BCP009_Total: 3093
Training size for Human_colon_16S7255677: 560
Training size for Human_colon_16S7255678: 900
Training size for Human_colon_16S8000484: 1046
Training size for Pan_T7935494: 2300
Training size for V1_Human_Lymph_Node: 3228
train size: 61826
valid size: 15469
86%|████████▌ | 344/400 [27:23<04:27, 4.78s/epoch, kl_loss=1.198, recon_loss=1147.656, val_kl_loss=1.229/0.233, val_recon_loss=1147.362/1143.838]
Early Stopping triggered
EarlyStopping counter: 30 out of 30
EarlyStopping counter: 344 out of 10
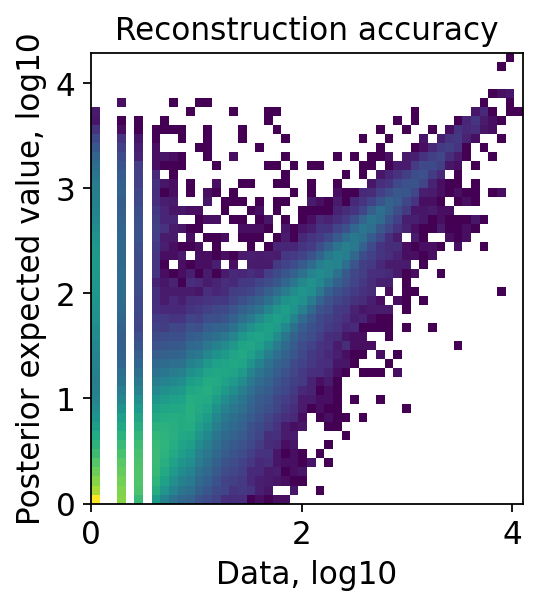
Examine QC plots.
[9]:
proc.impute(layer_key='counts', qc=True)
Get and check the co-embedding results¶
[10]:
adata = proc.adata
[11]:
sc.pp.neighbors(adata, use_rep='X_rep')
sc.tl.umap(adata)
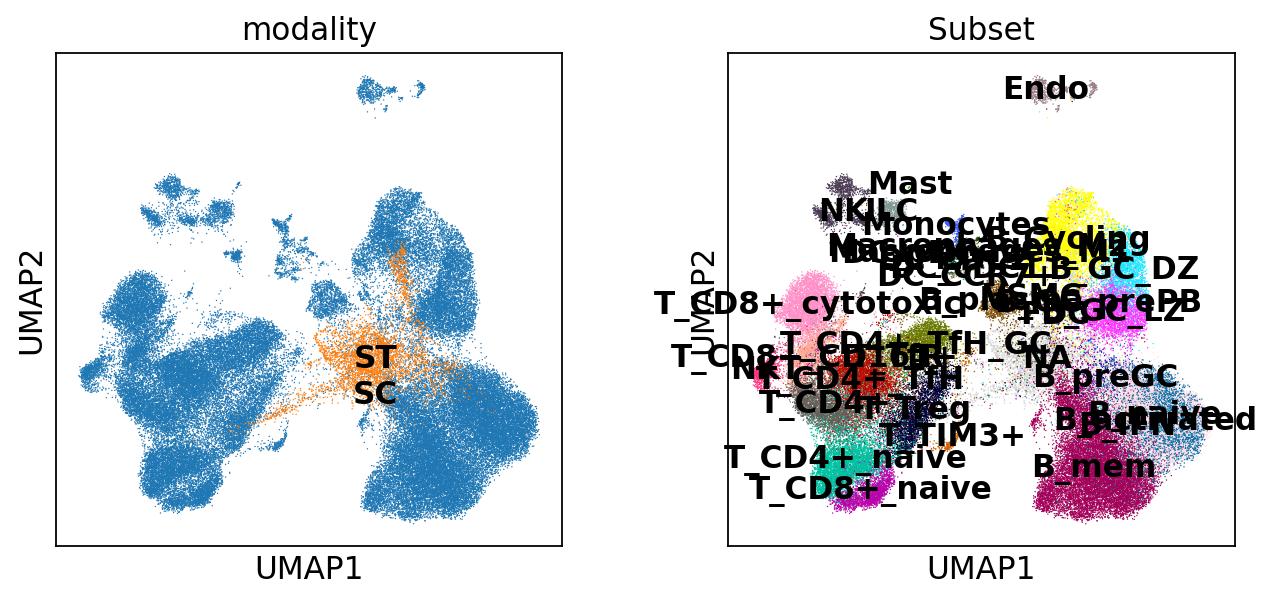
As we can see, two modalities manage to “mix” with each other in the left figure. Moreover, in the right figure, we can see that the structure of cell-types is totally preserved, indicating the integration(co-embedding) is biologically meaningful and the technical/batch effects are successfully eliminated.
[12]:
sc.pl.umap(adata, color=['modality', 'Subset'], ncols=2)
[13]:
torch.save(proc._functional.model.state_dict(), sc_pth)
Zonation after integration¶
Zonation, i.e. identification of spatial domains, is then conducted on the obtained embedding in the previous co-embedding process.
Note: This process required embedding produced in the previous process, so if the adata is not saved while the model(paramters) is saved, load the step instance proc and use proc.add_embed(key_added='<your desired key name>')
Training a spatial smoother(GCN) to spatially smooth the embedding extracted solely from transcprits. This training stage only involves the spatial data and is achieved in two sub-stage: 1. (Optional) Traning a spatial-data-specific decoder for the concentration on spatial data. This sub-stage still doesn’t utilize the spatial context, and is controlled by the following parameters: - epochs: #epochs, - batch_size: batch size, - n_dec_layers: #layers in decoder, - split_rate: the
proportion of data used as validation - use_st_decoder: whether to run this sub-stage, - decoder_type: the distribution to fit count data. 2. Training the spatial smoother to smooth the embedding of spatial data for zonation. This sub-stage is controlled by the following parameters: - n_glayers: #graph convolutional layers - smooth_epochs: #epochs in this sub-stage - tune_lr: learning rate to tune the st-decoder trained in the previous sub-stage, None means not tuning it.
[14]:
proc.generate_domains(epochs=800,
batch_size=1024,
n_dec_layers=1,
n_glayers=1,
split_rate=0.,
tune_lr=None,
smooth_epochs=800,
use_st_decoder=True,
decoder_type='nb')
==============Sub-Dataset Info==============
Batch key: Sample
Class key: Subset
Number of Batches: 24
Number of Classes: 34
Gene Expr: (4035, 2000)
Rep: (4035, 64)
Batch Label: (4035,)
============================================
100%|██████████| 800/800 [00:58<00:00, 13.63epoch/s, recon_loss=4178.889, val_recon_loss=-]
StDecoder trained
Start training smoother
100%|██████████| 800/800 [00:47<00:00, 16.79epoch/s, contrast_loss=5.846, recon_loss=4188.098, val_contrast_loss=-, val_recon_loss=-]
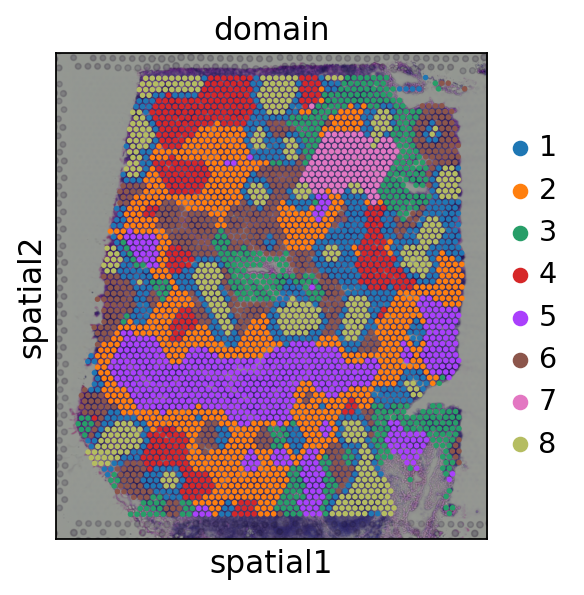
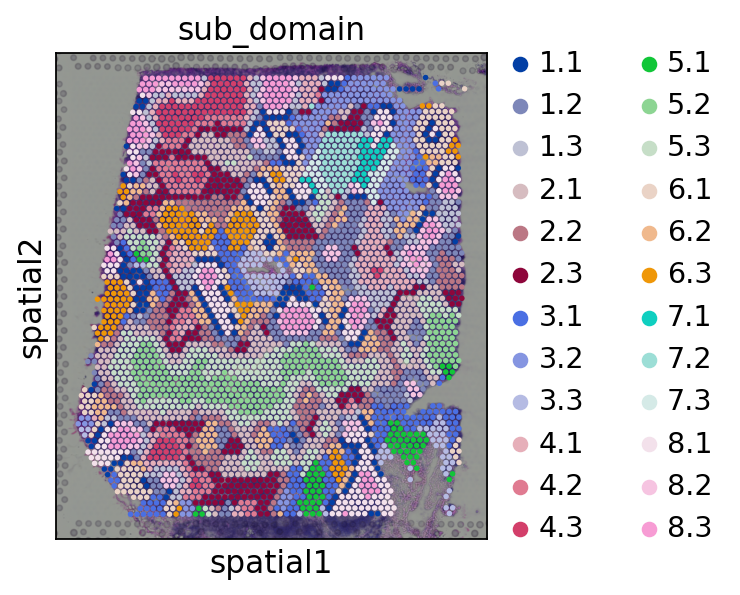
In defualt, the smoothed embedding will be stored in proc.st_adata.obsm['X_smoothed']. Here, we use KMeans to cluster the smoothed embedding and obtain 8 spatial domains.
Subdomains is identified by a further clustering based on spatial domains. We identifies 3 subdomains in each domain.
[15]:
proc.cluster(use_rep='X_smoothed', n_clusters=8)
sc.pl.spatial(proc.st_adata, color=['domain'], size=1.3,)
proc.sub_cluster(proc.st_adata,
pre_key='domain',
use_rep='X_smoothed',
n_clusters=3)
sc.pl.spatial(proc.st_adata, color=['sub_domain'], size=1.3,)
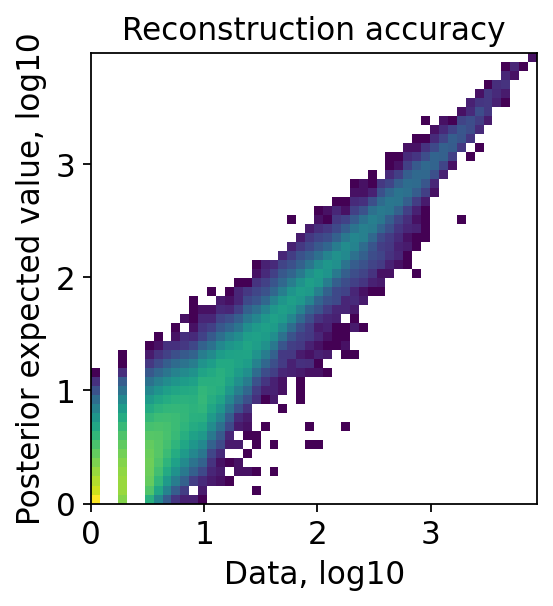
Examine the QC plots with original embedding and smoothed embedding through a same decoder(st-decoder if use_st_decoder is set previously)
[16]:
proc.st_impute(qc=True, smooth=False)
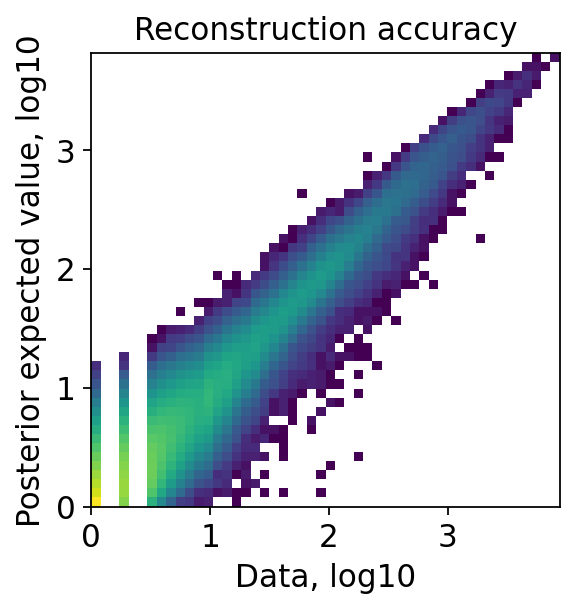
[17]:
proc.st_impute(qc=True, smooth=True)
Cell-type deconvolution with obtained embedding¶
STEP performs deconvolution at domain-wise since the spots in the same spatial domain share a similar cell-type composition. So this process requires two features: 1. Embedding of scRNA-seq and spatial data in the shared latent space obtained in the co-embedding process. 2. Spatial domains or subdomains stored in proc.st_adata.obs[<domain_key>].
The embedding is mainly used to infer the existance of a cell-type in a spot by evaluating the similarity in latent space between the corresponding embedding. Specifically, the embedding of scRNA-seq data is averaged by cell-types, denoted as anchors, and the similarity is measured by a sparse attention mechanism where “sparse” is to ensure the sparsity of cell-type occurence in a spot. To consider the individual effect in each spot, anchors are refined individually for each spot
through several(default 2) transformer encoders(can be disabled by setting hard_anchors=True). And to bound the refined anchors, we add a contrastive loss between refined anchors and original anchors as a regularization where the parameterT is the temperature in the contrastive loss.
The actual cell-type composition in a spot are inferred at transcript-wise by scaling the simlarity matrix inferred in the latent space. This is done by minimizing the negative log-likelihood with the “convolution” of estimated cell-type composition and cell-type signatures. Here the signatures can be obtained by averging the raw counts(set use_raw=True) or the decoder-imputed counts(set use_raw=False).
For the balance between multiple losses, use parameter rate to scale the two majority reconstruction lossess.
[18]:
proc.st_adata.obs['sub_domain_codes'] = proc.st_adata.obs['sub_domain'].cat.codes
proc.deconv(epochs=2000,
domain_key='sub_domain_codes',
domain_wise=True,
n_glayers=None,
use_raw=False,
hard_anchors=False,
T=0.1,
rate=0.01,)
100%|██████████| 2000/2000 [05:14<00:00, 6.36epoch/s, div=2.186, loss_ori=32.250, recon_loss=2080.224, sc_consistency=0.709, val_div=-, val_loss_ori=-, val_recon_loss=-, val_sc_consistency=-]
[19]:
st_adata = proc.st_adata
celltypes = proc.adata.obs['Subset'].cat.categories
prop_list = celltypes.to_list()
[20]:
st_adata
[20]:
AnnData object with n_obs × n_vars = 4035 × 2000
obs: 'in_tissue', 'array_row', 'array_col', 'batch_st', 'Sample', 'modality', 'n_genes', 'domain', 'sub_domain', 'sub_domain_codes', 'B_Cycling', 'B_GC_DZ', 'B_GC_LZ', 'B_GC_prePB', 'B_IFN', 'B_activated', 'B_mem', 'B_naive', 'B_plasma', 'B_preGC', 'DC_CCR7+', 'DC_cDC1', 'DC_cDC2', 'DC_pDC', 'Endo', 'FDC', 'ILC', 'Macrophages_M1', 'Macrophages_M2', 'Mast', 'Monocytes', 'NK', 'NKT', 'T_CD4+', 'T_CD4+_TfH', 'T_CD4+_TfH_GC', 'T_CD4+_naive', 'T_CD8+_CD161+', 'T_CD8+_cytotoxic', 'T_CD8+_naive', 'T_TIM3+', 'T_TfR', 'T_Treg', 'VSMC'
var: 'feature_types', 'genome', 'SYMBOL', 'mt_gene'
uns: 'spatial', 'domain_colors', 'sub_domain_colors'
obsm: 'spatial', 'mt', 'X_rep_tsfmr', 'X_smoothed', 'deconv', 'deconv_abundance'
layers: 'counts', 'st_expected_counts'
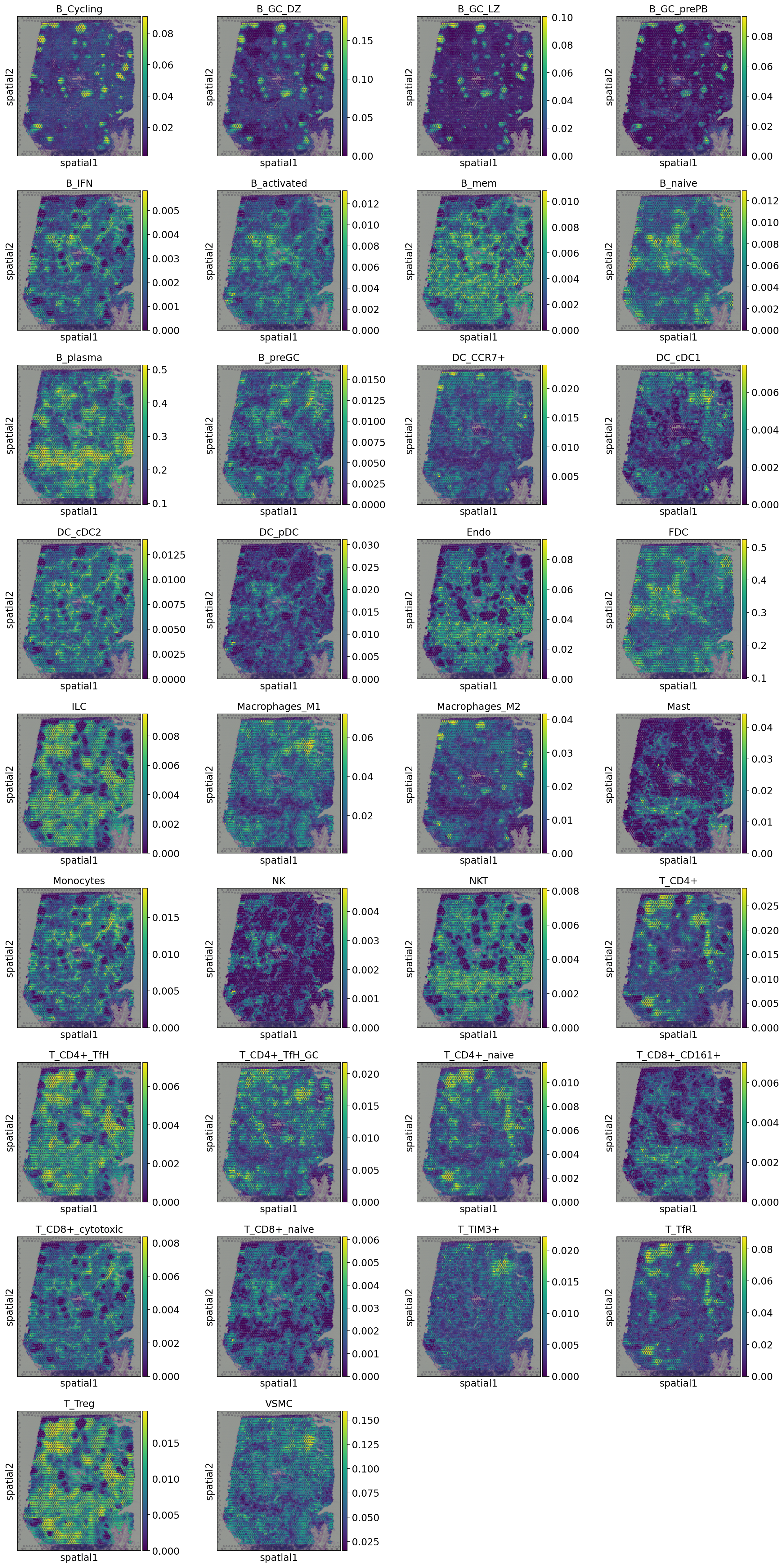
Visualize the inferred cell-type composition¶
[21]:
st_adata.obs[prop_list] = st_adata.obsm['deconv']
sc.pl.spatial(st_adata, size=1.3, color=prop_list,)
Perform spatially DE genes anlaysis¶
[22]:
adata_vis.obs['domain'] = st_adata.obs['domain'].astype(str).astype('category')
adata_vis.obsm['X_smoothed'] = st_adata.obsm['X_smoothed']
sc.tl.dendrogram(adata_vis, use_rep='X_smoothed', groupby='domain')
sc.tl.rank_genes_groups(adata_vis, groupby='domain', method='wilcoxon', use_raw=False)
[23]:
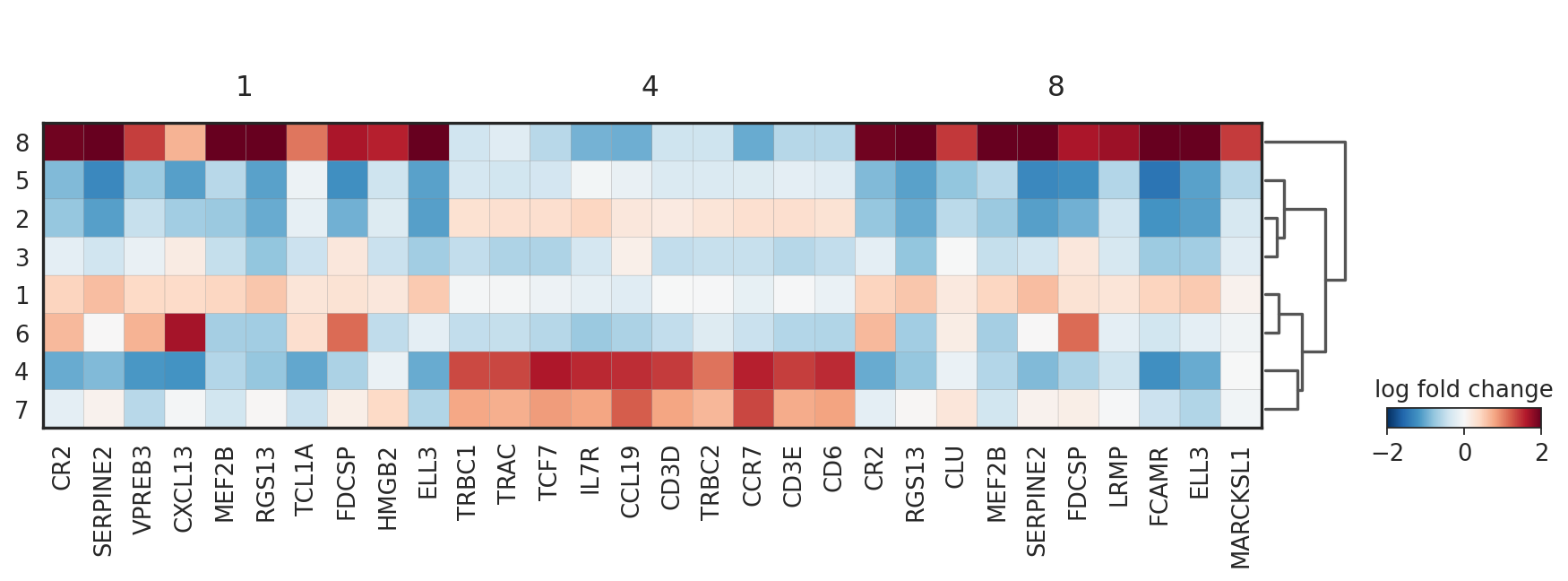
sc.pl.rank_genes_groups_matrixplot(adata_vis, groupby='domain', groups=['1', '4', '8'], values_to_plot='logfoldchanges', cmap='RdBu_r', vmin=-2, vmax=2)
WARNING: Groups are not reordered because the `groupby` categories and the `var_group_labels` are different.
categories: 1, 2, 3, etc.
var_group_labels: 1, 4, 8
[23]:
{'mainplot_ax': <AxesSubplot:>,
'group_extra_ax': <AxesSubplot:>,
'gene_group_ax': <AxesSubplot:>,
'color_legend_ax': <AxesSubplot:title={'center':'log fold change'}>}
[24]:
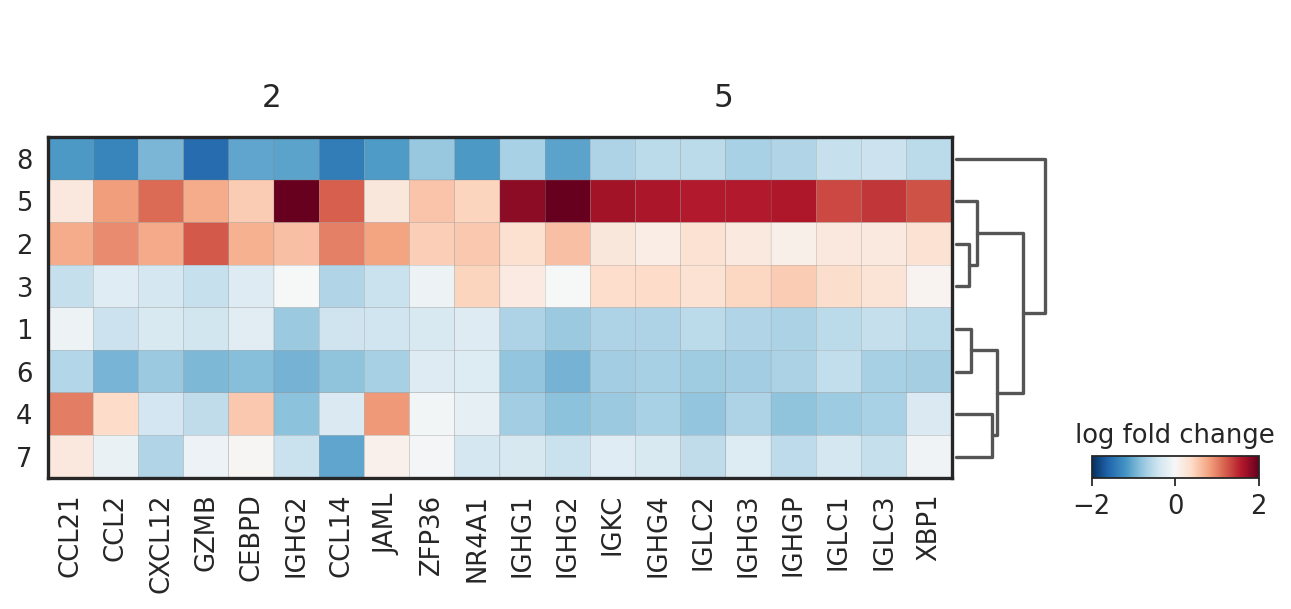
sc.pl.rank_genes_groups_matrixplot(adata_vis, groupby='domain', groups=['2', '5'], values_to_plot='logfoldchanges', cmap='RdBu_r', vmin=-2, vmax=2)
WARNING: Groups are not reordered because the `groupby` categories and the `var_group_labels` are different.
categories: 1, 2, 3, etc.
var_group_labels: 2, 5
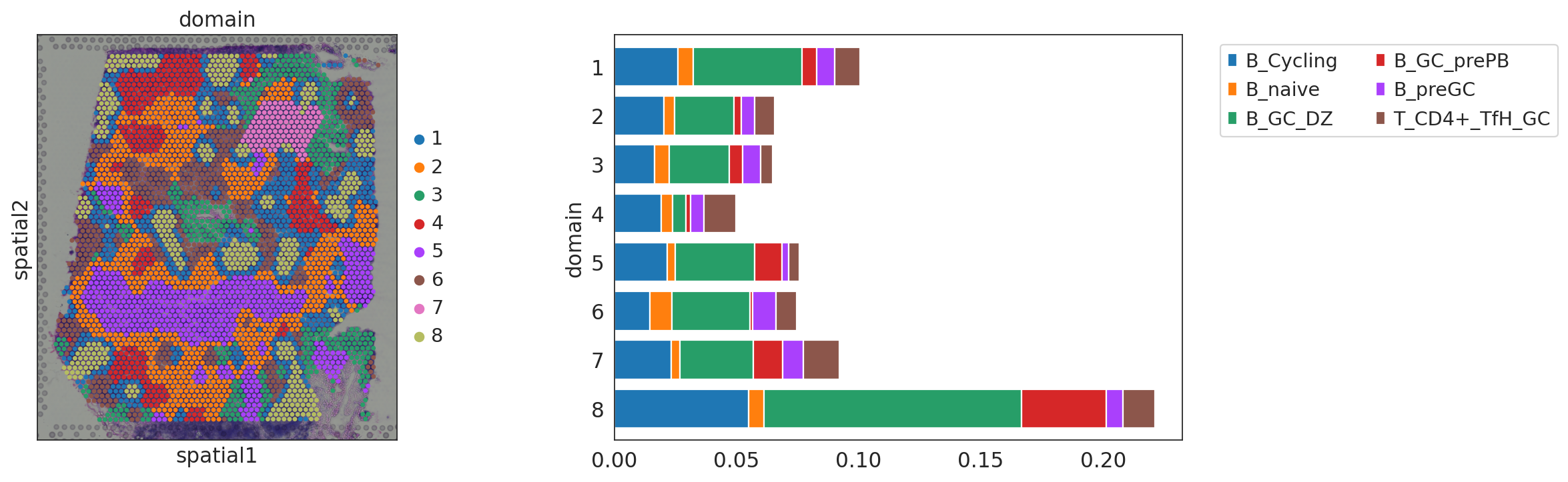
Summarize the average proportion of selected cell-types in each domain¶
[25]:
gc = ['B_Cycling', 'B_naive', 'B_GC_DZ', 'B_GC_prePB', 'B_preGC', 'T_CD4+_TfH_GC']
proc.summarize_domain(gc, adata=st_adata)